

|
Philosophy
|
Projects
|
Experience
|
Clients
|
Resume
|
Resources
|
© 1998-2014 Ulrick & Associates

Stable Isotope Ratios of Water
Stable isotope ratios are a powerful indicator of the source of a water sample because they are unchanged by most of the organic and inorganic chemical reactions in soil and rock.
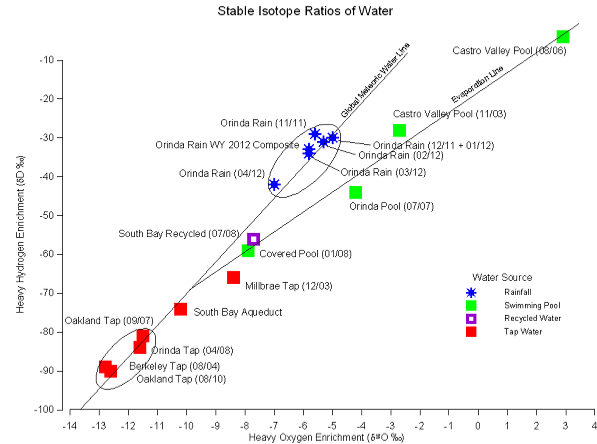
This graph illustrates the use of stable isotope ratios of water to determine the sources of water samples. The method is based on a linear mixing ratio between two endpoints of 1) local rainfall and groundwater derived therefrom and 2) imported tap water (Pardee Reservoir, Hetch Hetchy Reservoir, Delta). Bay Area water samples not affected by evaporation fall along the Global Meteoric Water Line between these two endpoints. South Bay Aqueduct and South Bay Recycled water are derived from mixtures of imported water and local surface water and groundwater. Swimming pool water and recycled water are affected by varying amounts of evaporation which changes the isotope ratios along an evaporation line.
The ratio of stable isotopes of H and O vary in precipitation as a function of temperature, elevation, and latitude. In California, extreme changes in elevation occur over relatively short distances. As storm systems move onshore from the Pacific Ocean the heavy isotopes 18O and 2H (or Deuterium, D) are depleted relative to the light isotopes 16O and 1H because condensation and precipitation selectively remove more of the heavy isotopes. Thus, surface water from mountain watersheds has less of the heavy isotopes 18O and 2H than water from coastal watersheds. For example, d18O in precipitation varies from approximately -4 ‰ along the Pacific coast to -15 ‰ in the Sierra Nevada.
Evaporation of surface waters, such as in a reservoir or swimming pool, depletes light isotopes and leaves behind a water that is enriched in the heavy isotopes. Old, evaporated swimming pool water is enriched in 18O and 2H can have an 18O along an evaporation line beginning from the isotope ratios of the source water.
Water is composed of hydrogen and oxygen (H2O). The atoms of hydrogen and oxygen that comprise water each occur as several different isotope-specific atoms or nuclides. A nuclide is defined by the number of protons, which defines the element, and the number of neutrons, which defines the specific isotope of that element. The atomic weight of a nuclide is the sum of the protons and neutrons. Most hydrogen (1H) has an atomic weight of 1 (1 proton and no neutrons). Heavy hydrogen or deuterium (2H or D) has an atomic weight of 2 (1 proton and 1 neutron). Most oxygen (16O) has an atomic weight of 16 (8 protons and 8 neutrons). Heavy oxygen (18O) has an atomic weight of 18 (8 protons and 10 neutrons).
Stable isotopes of water are measured as the ratios of the two most stable and abundant isotopes of each element. The stable isotope ratio of oxygen is the ratio of 18O (0.204 percent of all oxygen) to 16O (99.796 percent of all oxygen). Thus, the 18O/16O ratio is about 0.00204. Similarly, the stable isotope ratio of hydrogen is the ratio of 2H (0.015 percent of all hydrogen) to 1H (99.985 percent of all hydrogen) . Thus, the 2H/1H ratio is about 0.015.
Direct measurement of the absolute isotope ratios of elements is difficult. Instead, the difference in stable isotope ratio of a sample is measured relative to a known reference (Vienna Standard Mean Ocean Water). This difference is expressed using delta (d) notation. Because these differences are small, d values are expressed as parts per thousand (‰) differences from the reference. For example, a positive d value of +10‰ for oxygen (d18O = +10‰) means that the sample is enriched in 18O by 10 parts per thousand compared to the reference.
This graph illustrates the use of stable isotope ratios of water to determine the sources of water samples. The method is based on a linear mixing ratio between two endpoints of 1) local rainfall and groundwater derived therefrom and 2) imported tap water (Pardee Reservoir, Hetch Hetchy Reservoir, Delta). Bay Area water samples not affected by evaporation fall along the Global Meteoric Water Line between these two endpoints. South Bay Aqueduct and South Bay Recycled water are derived from mixtures of imported water and local surface water and groundwater. Swimming pool water and recycled water are affected by varying amounts of evaporation which changes the isotope ratios along an evaporation line.
The ratio of stable isotopes of H and O vary in precipitation as a function of temperature, elevation, and latitude. In California, extreme changes in elevation occur over relatively short distances. As storm systems move onshore from the Pacific Ocean the heavy isotopes 18O and 2H (or Deuterium, D) are depleted relative to the light isotopes 16O and 1H because condensation and precipitation selectively remove more of the heavy isotopes. Thus, surface water from mountain watersheds has less of the heavy isotopes 18O and 2H than water from coastal watersheds. For example, d18O in precipitation varies from approximately -4 ‰ along the Pacific coast to -15 ‰ in the Sierra Nevada.
Evaporation of surface waters, such as in a reservoir or swimming pool, depletes light isotopes and leaves behind a water that is enriched in the heavy isotopes. Old, evaporated swimming pool water is enriched in 18O and 2H can have an 18O along an evaporation line beginning from the isotope ratios of the source water.
Water is composed of hydrogen and oxygen (H2O). The atoms of hydrogen and oxygen that comprise water each occur as several different isotope-specific atoms or nuclides. A nuclide is defined by the number of protons, which defines the element, and the number of neutrons, which defines the specific isotope of that element. The atomic weight of a nuclide is the sum of the protons and neutrons. Most hydrogen (1H) has an atomic weight of 1 (1 proton and no neutrons). Heavy hydrogen or deuterium (2H or D) has an atomic weight of 2 (1 proton and 1 neutron). Most oxygen (16O) has an atomic weight of 16 (8 protons and 8 neutrons). Heavy oxygen (18O) has an atomic weight of 18 (8 protons and 10 neutrons).
Stable isotopes of water are measured as the ratios of the two most stable and abundant isotopes of each element. The stable isotope ratio of oxygen is the ratio of 18O (0.204 percent of all oxygen) to 16O (99.796 percent of all oxygen). Thus, the 18O/16O ratio is about 0.00204. Similarly, the stable isotope ratio of hydrogen is the ratio of 2H (0.015 percent of all hydrogen) to 1H (99.985 percent of all hydrogen) . Thus, the 2H/1H ratio is about 0.015.
Direct measurement of the absolute isotope ratios of elements is difficult. Instead, the difference in stable isotope ratio of a sample is measured relative to a known reference (Vienna Standard Mean Ocean Water). This difference is expressed using delta (d) notation. Because these differences are small, d values are expressed as parts per thousand (‰) differences from the reference. For example, a positive d value of +10‰ for oxygen (d18O = +10‰) means that the sample is enriched in 18O by 10 parts per thousand compared to the reference.
